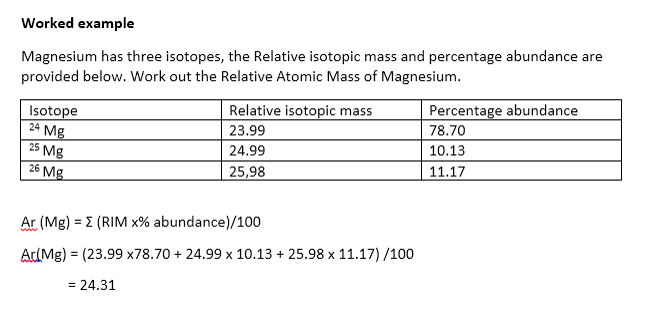
8 Explain the Difference Between Percent Abundance and Relative Abundance
In relative grading of students the grades are set according to the highest marks for a paper. The relative abundance is just a number.
Element X Has Two Isotopes Of 16 And 18 Its Relative Atomic Mass Is 16 4 What Is The Percentage Abundance Of Each Isotope Quora
This average mass is the atomic mass of candium.
. This average mass is the average atomic mass of Beanium. Up to 24 cash back 4. Relative Mass Relative Abundance x Average mass 5.
Compare the total values for Steps 3 and 6 on Table 61. 329 0145 0477g ----------- 142g IX. What is the result.
What does relative abundance tell you. Think about a paper where the highest mark is 55. Multiply the relative abundance from step 8 by the average mass of each isotope to get the relative mass of each isotope.
Relative abundance makes reference to the proportion of the elements in a group with relation the total number of elements used. Explain the difference between percent abundance and relative abundance. Explain the difference between percent abundance and relative abundance.
Explain why the difference would be smaller if larger samples. What does relative abundance tell you. So we can get a meter stick measure the length of each and add them to get a total.
Explain any differences between the atomic mass of your vegium sample and that of your neighbor. Why cant atomic masses be calculated the way the total for row 3 is calculated. If there are two isotopes of element B and the percent abundance for one isotope is 43 then 43 of that element on earth is that isotope.
Can make them move up or down on the y axis as she chooses. Calculate the percent abundance of each isotope by multiplying the answers you got in number 2 by 100. Abundance of atoms for each isotope x 100 of atoms for all isotopes 3.
The percent abundance is the percent value of the relative abundance. View the full answer. Unlike in absolute grading where a grading system already exists in relative grading the grades awarded depend on the marks gained by the best students.
Relative abundance is the percent abundance as a decimal. The percent is just the relative abundance on the meter stick scale divided by the total of all the peaks and. Then we can do the percent of each.
What does relative abundance tell you. What is the result when you total the individual relative abundances. The percent abundance of each kind of candy tells you how many of each kind of candy there are in every 100 particles.
Relative abundance X 100 4. 5 Calculate the atomic mass of all Beanium particles by adding the relative masses. Total number of specific candytotal number of candy 3.
113 0164 0185 g Pretzel. Relative abundance 100 Plain. Divide the percent abundance from step 6 by 100 to get the relative abundance of each isotope.
6 Explain the difference between percent abundance and relative abundance. Calculate the relative abundance of each isotope Relative Abundance Percent abundance basically the expressed as a decimal 100 4. SHOW ANSWER Relative means you take the required amount from the actual amount which gives you the abundance and its relative because you compare it to the required amount.
0164 100 164 Pretzel. Explain the difference between percent abundance and relative abundance. Calculate the relative mass for each isotope.
A newly discovered element consists of 2 isotopes one with a mass of 95 and a relative abundance of 172 and another with a mass of 22 and a relative abundance of 828. In fact the operator of the M. Calculate the average mass of all candium particles by adding the relative masses.
Abundance abundancerequired amount X 100 Thanks Useless. 109 0691 0735g Crispy. This is good for a hard paper.
4 Calculate the relative mass of each isotope by multiplying its relative abundance from step 3 by its average mass. 0145 100 145 4 --------- 100 8. Explain why the difference would be smaller if larger samples.
Explain any differences between the atomic mass of your vegium sample and that of your neighbor. Calculate the relative abundance of each isotope by dividing the number of particles by the total number of particles in the candium element. Percent abundance is the percentage of the element that is that particular isotope.
What is the result when you total the individual percent abundances. What is the result when you total the individual percent abundance values for each isotope. What is the result when you total the individual relative abundance values for each isotope 4.
Average mass relative abundance relative weight Plain. Write the result in the Percent Abundance row. Place these values in the Relative Abundance row.
Compare the total values for Steps 3 and 6 on Table 5-1. The individual percent abundances. Why cant atomic masses be calculated the way the total for row 3 is calculated.
Explain the difference between percent abundance and relative abundance. Difference between percent abundance and relative abundance. How does the.
Determine the atomic weight of the new element. 0691 100 691 Crispy. Compare the total values for rows 3 and 6 in the data table.
The percent abundance is the relative abundance as a percentage of the required amount. Calculate the relative mass of each isotope by multiplying its relative abundance from Step 3 by its average mass.

Isotopic Abundance Example Youtube
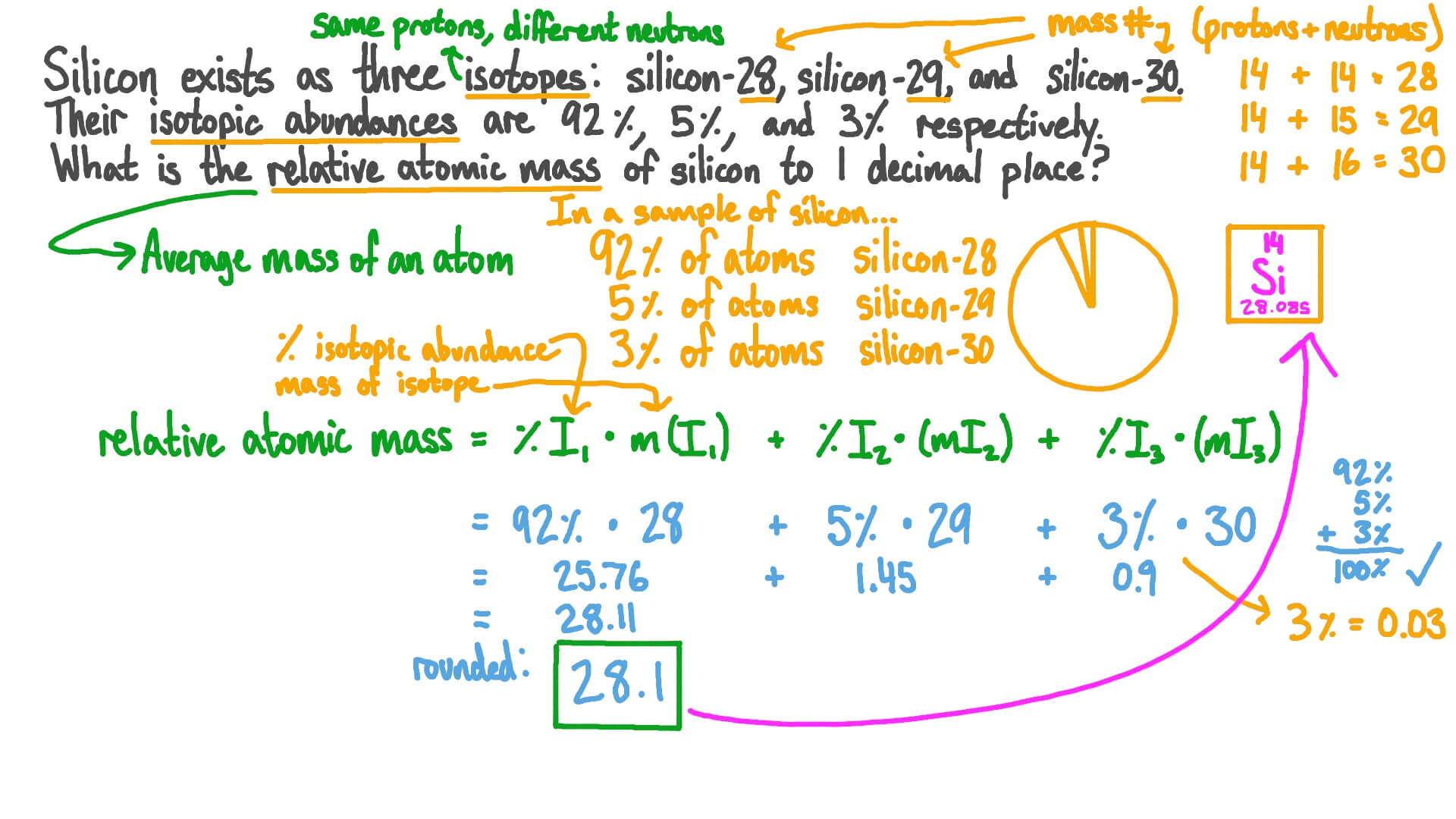
Question Video Using Relative Abundance Of Isotopes To Calculate Relative Atomic Mass Nagwa
Comments
Post a Comment